Isotope Fractionation of Uranium in the Process of Leaching of Nuclides of
Dispersed Fuel of RBMK of the Chernobyl NPP
””
German N. Bondarenko and Emlen V. Sobotovich
””
State Scientific Center of Environmental Radiogeochemistry NAS,
Palladin av. 34, 252680 Kiev, Ukraine, tel. (044) 444-12-15, fax (044)444-00-60
””
SUMMARY
The particles of fragmented nuclear fuel containing uranium of different degree of oxidation were found as the parts of conglomerates in the premises of the IV unit and in soils of the Chernobyl NPP Industrial site. When leaching uranium from contaminated soils, fractionation of the nuclides of 235U and 238U between solid and liquid phases may take place. Fractionation was not indicated in leaching of uranium from the particles patched into graphite fragments.
Fractionation of uranium nuclides is due to difference in energies of these nuclides bonds in crystal lattice of solid phase of the products of fuel fragmentation. Two hypotheses are accepted. The first one supposes heterogeneous composition of nuclear fuel of RBMK reactor consisting of fractions with different enrichment by 235U, uranium isotopes of the more enriched fraction having higher mobility due to features of preparation technology. Another hypothesis bases on the assumption of selective loosening of bonds of separate 235U atoms takes place in the solid body under action of slow neutrons.
””
INTRODUCTION
Works on study of uranium isotopic composition in the products of accidental ejection from the Chernobyl NPP by its scope take up rather modest place among other studies on consequences of 1986 accident in spite of obvious connection between 235U distribution in the process of fuel destruction and nuclear safety of Sarcophagus. Studies on the uranium isotopes distribution in dissolution, leaching of dispersed fuel gave discrepancy results: some experiments showed 235U enrichment of uranium in water solutions relative to the solid phase [1,2], others showed an identity of the isotopic composition of the both of phases [2-4]. The results were explained by the heterogeneous composition of fuel component of Chernobyl ejection. Detailed analysis of the performed studies has enabled to establish the regular connection between the possibility of uranium isotopes fractionation and conditions of dispersed fuel formation and to propose the version of rise of weakening of 235U bond in the solid phase of fragmentation products of fuel.
””
1. Fuel-containing products of accident on the IV unit core of Chernobyl NPP.
Unit cores of the Chernobyl NPP are equipped with reactors of channel type (RBMK), that use graphite as reaction moderator and light water as heat-carrier. Reactor core is an vertical cylinder 11.8 m in diameter and 7 m in height. It contains fuel assemblies, moderator, heat-carrier, technological channels, neutron absorber rods. Graphite brickwork makes up vertical columns with through holes for placement of technological and control channels. Graphite density is 1.65 g/cm3. Fuel assembly consists of two sequentially connected sections 3.5 m each in length. Each section includes central tube from zirconium alloy, steel distanced grid, 18 fuel rods.
Initial enrichment of fuel is 2%. If the design burn-up is taken as 18.6 MW/ton, then nuclide contents are: 235U ? 4.1; 236U ? 2.1; 238U ? 969.3; 239Pu ? 2.4; 240Pu ? 1.7; 241 Pu ? 0.5; and 242Pu ? 0.3 kg per tone of initial uranium [5]. So spent nuclear fuel should contain 0.42 % of 235U.
On the moment of the accident the reactor active zone of IV block of the Chernobyl NPP contained 1659 fuel assemblies (FA). Their nuclear fuel total mass was 190.2 tons [4]. The bulk of the FA was enclosed in the first charge cassettes with the burn-up of 11 to 15 MW× day/kg of U. Some amount of ”Čfresh”É nuclear fuel was in the active zone too. Calculations of mass and activity of fission products and transuranium elements that took account of factors of irregularity of the field of energy emission in RBMK-1000 were carried out for each cassette. The total mass of each accumulated nuclide was estimated by summing over all fuel assemblies in the reactor core. According to these estimates 235U content conformed to effective enrichment of 1.1% [2]. Plutonium nuclides contents were estimated in: 239Pu ? 2.166, 240Pu ? 0.925, 241Pu ? 0.257, and 242Pu ? 0.074 kg per ton of uranium [2-1].
More than 180 tons of nuclear fuel are considered to be enclosed in the object ”ČUkrytiye”É (”ČSarcophagus”É). Irradiated fuel inside ”ČSarcophagus”É has the following modifications:
- fragments of the reactor core - fuel tablets, fuel assemblies and their fragments, radioactive graphite, fragments of technologic channels;
- finely dispersed fuel - ”Čhot”É fuel particles that were the main radioactive components of dispersed phase of aerosols and solid precipitates on different surfaces;
- lava-like masses containing nuclear fuel;
- crystalline nova-formations that are bright-yellow spots and figures;
- water solutions.
Finely dispersed fuel of micron dimensions was characterized form of accidental ejection of the Chernobyl NPP into the environment. In the early months and years after the accident they were the objects of numerous studies. ”ČHot”É particles were sampled in the premises of the IV unit, in close proximity to the Chernobyl NPP, on the area of the Chernobyl NPP industrial site and sanitary zone, on the territories of Ukraine, Byelaruss as well as Scandinavian countries, Central and West Europe.
The most of works were limited by studies of radionuclide content of particles. Studies concerning with radionuclide state in particles are not numerous. Works on study of chemical and structural composition of particles, on kinetics of dissolution and leaching of radionuclides, and on distribution of radionuclides in the system ”Čsolid phase - solution”É [1-3, 12-14] are among them.
””
2.
Elemental and structural composition of hot particles.Some particles contained radioactive substances that had low boiling temperature (131I, 137Cs and 134Ñs), others contained radionuclides with rather high boiling temperature (isotopes of Sr, Ce, Zr, Pu, Am, Cu, Np etc.) as the parts of uranium matrix [3]. At morphology fibre-like and spherical microconglomerates and that of irregular habitus. Density of the heaviest of the particles reached 8 g/cm3 [2, 6].
In one of samplings hot particles of two varieties - 30 m m and 1 to 2 m m in size - predominated. Particles of the first variety were identified as fuel ones, those of the second variety were recognized to be condensational particles formed during burning of graphite. In the sampling of particles that deposited in the premises of ”ČSarcophagus”É maximum of dimension distribution was in the region of 15-20 m m [6].
At a distance of more than 1 km from the accident unit, separate hot particles were found on the surface of the earth, buildings and constructions as the parts of conglomerates of particles of different substantial composition. Oxides of U(IV) and U(VI) as well as blobs of carbonaceous matter, small fragments of quartz, aluminosilicate matter, ferrous minerals, rounded titanic fragments were detected in these conglomerates by the methods of microscope probing and X-ray-structural analysis [19].
Sampling of hot particles from the soils in the floodplain of the Prypiat (5-7 km distant from the unit) and those from soddy-podzolic soils from st.Yanov (2-3 km from the unit) was presented by microconglomerates and fragments of reactor graphite [2]. Several types of particles can be separated by their composition and structure:
- quartz-feldspar, clastogeneous, having grains predominantly of sharp-angle profile cemented by micaceous-kaolinic mass;
- clastogeneous substantially micaceous-silicate, consisting of entangled fibrous aggregates of sheet crystals;
- condensation-clastogeneous, consisting of microgeodes, spherules of condensation genesis with admixture of clastogeneous matter.
Predomination of small sharp-angle clastic fragments, cementation, presence of implanted uranium particles, condensation formations have enabled to assume that the microconglomerates as the radioactivity carriers were formed mainly in result of agglutination of particles of fluid materials used during fire fighting, dispersed materials of reactor unit constructions, fuel and graphite at high temperatures [10, 12, 15].
One of the fragments, 2x3 mm in size, sampled in the premise of the IV unit consisted of grains of graphite and small fragments of concrete. Grains of quartz and microcline were cemented with fine Ca-containing material. Particles with high atomic mass that had substantially uranium composition were found in that fragments by vision of preparates in reverse reflected electrons. Uranium particles of 1-50 m m in size were placed on the surface of graphite aggregates or were slightly implanted into them.
Two types of particles were separated by the morphology:
- shperules and their fragments of 3-90 m m,
- irregular-shaped particles, predominantly sheet-shaped, with dimensions of fractions of micron to 50 m m.
Spherical particles showed microdifraction picture corresponding to UO2 structure with diminished parameter of elemental cell (à0 = 0.535 ± 0.005 nm). It was attributed to the partial oxidation of U(IV) to U(VI), that increased number of defects in crystals and decreased effective dimensions of blocks of coherent scattering of electrons. Mixture of uranium dioxide and elemental uranium was identified by characteristic X-ray emission in number of plate-shaped particles. Hypothesized process of UO2 sublimation at high temperatures in the authors view could be accompanied by formation of pre-stoichiometric oxide UO2?«Ų and following disproportionation to UO2 and U.
Among the synthesized uranium oxides used as a nuclear fuel in the modern energetic units the series of phases were established that transfer one into another from UŖĀ2 to UŖĀ3 gradually or abruptly as uranium oxidation occurred from U(IV) to U(V) and U(VI).
Synthetic UŖĀ2 oxide has cubic crystal structure with elemental cell dimension of 0.548 nm. As uranium oxidation occurs, at first diminishing of this dimension that is recognized as a result of isomorphous replacement of U(IV) (ion radius is 0.1 nm) by U(VI) (r=0.08 nm), and than decreasing of oxide symmetry to tetragonal U4Î9 are observed. More oxidized phase U3Î8 is known to have two polymorphous modifications (rhombic and hexagonal), maximally oxidized phase UŖĀ3 - five modifications, one of them is amorphous. Oxidation series consists of the next discrete phases:
U4Î9 ? U3Î7 ? U2Î5 ? U3Î8 ? UŖĀ2 ? UŖĀ2.25 ? UO2.33 ? UŖĀ2.5 ? UŖĀ2.7 ? UŖĀ3.
In the sampling of particles aspirated from the aerosols from the object ”ČSarcophagus”É about 10% of the total activity were contained in fraction < 1 m m, about 70% of it - in fraction 1-5 m m, and about 20% of activity were concentrated in the rough-dispersed fraction. The main input into activity of the dispersed matter deposited on the surfaces fraction 1-10 m m has made (~70%), rough-dispersed fraction (>10 m m) contained about 20% of the total activity, fine-dispersed one - about 10% [8]. The finest particles sampled from the soils of the south Byelaruss had the highest specific activity [15].
All the long-lived products of nuclear fuel fission were detected in the dispersed phase of aerosols. 238Pu and 239+240Pu were found in all the separated fractions of dispersed phase of aerosols. 238Pu /239+240Pu ratios were close to those of the ”Čmean”É nuclear fuel of the IV unit. There was no correlation between 144Ce and 241Am [4,7]. Activities of 144Ce in the separate particles of the sampling of 17 to 55 m m in dimension were (8.1 - 9.6) ´ 109 Bq/g that was close to ”Čmean”É radionuclide content in the fuel at the moment of the accident.
Rather large-sized spherical particles up to several millimeters in diameter were collected on the surfaces of steam-dumping valves in the steam-distributing premise of the accidental unit [6]. Study of radionuclide distribution with depth in these particles showed the maximal contents of plutonium as well as radioactive isotopes of Sr and Cs in the outer layers and decreasing of their activities up to the centers of particles. Plutonium contents in the upper layers of spherical particles were from 1´ 105 to 1.5´ 105 Bq/g when mass concentrations of uranium were of 0.5 to 7.5%. Plutonium contents in other layers of the particles were less by some factors and in some cases by several orders of magnitudes. 238Pu/ 239+240Pu ratios were stable in all these cases and corresponded to those of the ”Čmean”É nuclear fuel of the IV unit.
Above-mentioned data testify that the processes of high-temperature atomizing of elements and subsequent spontaneous solid condensation of vapour resulted in the formation of spherical particles, proceeded in the active stage of the accident at least in some point of the IV unit. Recrystallization of the nuclear fuel material accompanied with establishing of contents of Pu, 144Ñe, 241Am, 90Sr, and U in different layers of spherical particles that rather differed by their ratios from the ”Čmean”É nuclear fuel of the IV unit. The growth of fiber-like particles proceeded obviously in the active stage of the accident too from the gas phase at the temperatures higher than 3,000 0Ñ.
””
3. Leaching of fission products from hot particles in soils.
Radioactivity of soils in the industrial site of the Chernobyl NPP was attributed to fallout of irradiated fuel in the next principal forms:
In the first months after the accident more than 99% of uranium and plutonium radionuclides were accumulated in the upper 2 cm-layer of the soil in the nearest zone of the Chernobyl NPP (Table 1).
Table 1. Vertical distribution of plutonium and 238U/235U atomic ratios in total uranium in the soils of the nearest zone of the Chernobyl NPP.
|
Index |
239(240) Pu contents in layers, % |
238 U/235U atomic ratios |
||||||||
|
0-1cm |
1-2 cm |
2-3 cm |
3-4 cm |
4-5 cm |
0-1 cm |
1-2 cm |
2-3 cm |
3-4 cm |
4-5 cm |
|
|
1 |
92.0 |
7.4 |
0.4 |
0.2 |
- |
79.9 |
128.2 |
134.7 |
137.4 |
137.6 |
|
2 |
91.2 |
7.9 |
0.6 |
0.2 |
0.1 |
80.3 |
129.6 |
129.6 |
137.3 |
137.7 |
|
3 |
95.9 |
3.8 |
0.3 |
- |
- |
89.6 |
135.8 |
137.3 |
137.8 |
137.8 |
|
4 |
96.7 |
2.9 |
0.3 |
0.1 |
- |
96.1 |
126.7 |
134.8 |
137.1 |
137.6 |
|
5 |
91.8 |
7.8 |
0.3 |
0.1 |
- |
98.4 |
131.5 |
136.9 |
137.8 |
137.8 |
|
6 |
96.0 |
3.4 |
0.4 |
0.1 |
0.1 |
103.5 |
132.9 |
137.1 |
137.6 |
137.6 |
Vertical distribution of the fission products in the first weeks and months after the accident was the same. Their activity was localized in form of hot particles, that was confirmed by the autoradiography data [15].
In soils hot particles are exposed to destruction due to effects of soil solutions and products of bacteria action. In the result of leaching of radionuclides and dissolution of matrix, products of uranium fission were carried out from the particles and their water-soluble compounds were formed. This process and following transformations of radionuclide forms can be presented by processes of mobilization, immobilization, remobilization of radionuclides:
W
k3
E
””
A0 = Ap + W + E + F
where : Ŗ¢0 is bulk radionuclide activity; Ŗ¢p - part of radionuclide in solid-phase particles; W - part of radionuclide in water-soluble form; Ŗ© - part of radionuclide in ion-exchangeable form; F - part of radionuclide in fixed form.
Mobilization is identified with carry-out of radionuclides from solid-phase particles and formation of water-soluble and ion-exchangeable forms of radionuclides that are subjected to water migration. Immobilization is connected with formation of relatively conservative (fixed) forms of radionuclides. Remobilization is an opposite process to immobilization.
According to deterministic kinetic model of transformation [14], activity of the mobile form of radionuclide (AM) is :
k1 - k3
AM = A0 {-----------------[(exp(-k1t) - exp(-k2 - k3)t]
-k1 + k2 + k3
k3
+ ---------- [1 - exp(-k2 - k3)t]}
k2 + k3
where AM and A0 - activities of radionuclide mobile and initial forms, kn - constants of rate of mobilization, immobilization, and remobilization of radionuclides sequentially.
The constant of radionuclide mobilization rate k1 is a characteristic of the resistance of solid-phase fallout in the soil. Its value first of all depends on the solid-phase particles qualities (material composition, degree of dispersion, way of formation) as well as on physical-chemical characteristics of the soil. The constants of radionuclide immobilization rate k2 and remobilization rate k3 are determined by radionuclide chemical properties, and for each radionuclide they essentially depend on physical-chemical characteristics of the soil. Numerical values of constants of transformation rates of 90Sr and 137Ñs forms were estimated by calculations on the base of comparison of the data on radionuclides forms in the soils in the different moments of post-accidental period.
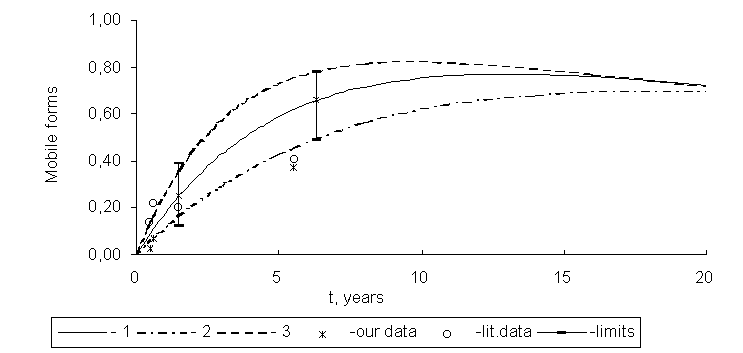
The kinetic curves of 90Sr and 137Ñs mobile forms accumulation in soddy-podzolic soils in the Chernobyl exclusion zone as well as experimental data on their contents are presented in Figs. 1 and 2.

Fig. 1. Kinetic curves of 90Sr mobile forms accumulation in soddy-podzolic soils in the exclusion zone:
1 - k1 = 0.195, k2 = 0.02; 2 - k1 = 0.12, k2 = 0.02; 3 - k1 = 0.30, k2 = 0.02 year-1.
””
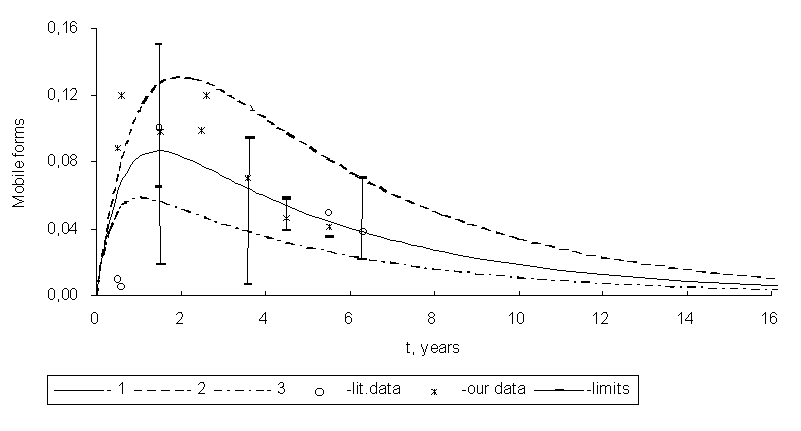
Fig. 2. Kinetic curves of 137Cs mobile forms accumulation in soddy-podzolic soils in the exclusion zone:
1 - k1 = 0.195, k2 = 1.7; 2 - k1 = 0.195, k2 = 1.0; 3 - k1 = 0.195, k2 = 2.7 year-1.
In the first years after the accident, sharp increasing of 90Sr mobile forms content took place, and then their stabilization was observed. So immobilization was not substantial in 90Sr forms transformation. Dynamics of changes of 137Cs mobile forms activities in soddy-podzolic soils was characterized by maximum that was observed in 1987-1988 and subsequent decreasing of ion-exchangeable form content at the expense of advancing immobilization.
The mean value of constant of radionuclide mobilization rate was evaluated in 0.195 year-1 with variations of 0.12 to 0.30 year-1. Hot particles that were in the air-dry state preserved fission products in their compound for a much longer time.
””
4. Mobility of uranium isotopes in the soils containing hot particles.
The radionuclide status in solid substances is commonly studied by leaching procedure and research of its distribution between different phases and chemical forms. Solutions that don”Ēt dissolve matrix matter are used. So admixtures elements residing out of nodes of crystal lattice are leached. Nuclides of daughter radioactive elements are leached from uranium and thorium minerals. Atoms resided inside a crystal or in the narrow capillaries migrate to the crystal surface in the result of diffusion over vacancies, internodes and capillaries. Desorption of the element from the surface depends on the concentration of displacing element in the solution and proceeds to attainment of equilibrium. Parent radionuclides move into solution only in the result of destruction of crystal lattice, i.e. dissolution of the matrix matter.
Mobility of uranium isotopes and fission products in soils contaminated by solid-phase accidental fallouts was evaluated by leaching method in series of works [1-3, 14, 15]. For these purposes sequential leaching of nuclides from solid phase by water solutions of salts and acids was performed.
Experiments on leaching of uranium isotopes from the top layer soils, which contained hot particles, were carried out at the laboratory of transuranium elements geochemistry of Department of Environmental Radiogeochemistry of Institute of Geochemistry and Physics of Minerals [14]. Soils were sampled in 1986 mainly at the industrial site of Chernobyl. Determinations of atomic (mass) ratios such as 238U/235U and 235U/236U were carried out by the MI-1320 and MI-1201T mass-spectrometers with 0.2% limiting relative error. Alpha-spectrometric determinations of 234U/238U activity ratios as well as plutonium activity were carried out with Si (Au) - detector within a 2% error. Content of uranium in the soil was estimated by the mass-spectrometric isotopic dilution method within a 0.8% error. Standard 235U solution with 238U/235U isotope ratio equal to 0.1022 was used as an isotopic label. Limiting relative errors for all the experimental measurements were estimated within 95% confidence interval.
To check of the obtained 238U/235U ratios, a series of duplicate analyses were performed at the mass-spectrometric laboratory by Prof. N.P.Tsherback, which confirmed validity of the previous results.
Extraction of uranium from the soils was carried out in these experiments with concentrated hydrochloric acid. After solid phase separation isolation of the uranium from the solution and its preparation to mass-spectrometric analysis was performed. Experimental data on isotopic composition of uranium and its content in liquid phase are presented in Table 2.
Table 2. Uranium isotopic composition and content in soils in the near-field zone of the Chernobyl NPP [1].
|
No |
Soil sampling point |
238 U/235U * |
235 U/236U * |
234 U/238U** |
238 U (m g/g) |
235 U,% |
|
1 |
Site of the IV unit |
39.68 |
16.08 |
3.06 |
1.38 |
2.46 |
|
2 |
North-western part of the cooling pond |
44.82 |
26.12 |
2.75 |
0.796 |
2.18 |
|
3 |
Vicinity of Yanov railway bridge |
51.21 |
14. 28 |
2.38 |
1.173 |
1.92 |
|
4 |
Left bank of the Prypiat |
53.3 |
8.16 |
2.00 |
0.745 |
1.84 |
|
5 |
Yanov railway station |
52.6 |
13.05 |
3.96 |
0.910 |
1.87 |
|
6 |
Red forest |
57.8 |
26.77 |
2.32 |
0.848 |
1.70 |
|
7 |
v. Yanov |
62.8 |
35.72 |
2.57 |
0.764 |
1.57 |
|
8 |
v. Leliv |
77.5 |
83.2 |
2.39 |
1.092 |
1.27 |
|
9 |
Paryshev channel |
93.8 |
140. 3 |
3.22 |
0.632 |
1.06 |
|
10 |
v. Burakivka |
102.4 |
47.26 |
2.18 |
0.826 |
0.97 |
|
11 |
v. Tolsty Lis |
106.3 |
76.6 |
1.94 |
0.911 |
0.93 |
|
12 |
town of Prypiat |
108.4 |
92.4 |
1.82 |
2.14 |
0.91 |
|
13 |
Chernobyl |
112.9 |
103.7 |
1.86 |
0.948 |
0.88 |
|
14 |
v. Cherevach |
126.3 |
194.8 |
1.69 |
1.013 |
0.79 |
*
Ratios between mass concentrations of uranium isotopes;**
Ratios between activities of uranium isotopes.””
Obtained data on the uranium contents in soils did not exceed the Clark values varying from 0.632 to 2.14 m g/g. This testified at least to comparability of technogeneous uranium content in soils to the natural one. The 238U/235U ratio in the uranium extracted from the soils varied from 44.82 to 126.3; 235U/236U ratio varied at more greater extent. Shifts in the isotopic composition relative to natural and even to technogeneous uranium were pronounced: 238U/235U isotopic ratios for some leachate-solutions were found to be considerably lower than that of the initial fuel.
Besides, dependence of uranium isotopic composition in soils upon their distance from the source was observed: uranium enrichment by 235U isotope increased with approaching to the IV unit, i.e. with enrichment of soils by technogeneous (fuel) uranium.
Mobility of uranium isotopes in the solid phase was evaluated by the method of their sequential leaching in the variable physico-chemical conditions: different acids with admixtures were used (Table 3). It was found that the 238U/235U ratio for the first leachates ranged from 15.6 to 61.4, whereas in the case of more complete extraction of uranium from the same soils it varied from 44.8 to 93.8. On these grounds the authors of the work assumed that the reduction in the 238U/235U isotopic ratio in solutions might be explained by the fact that the technogeneous fuel uranium (or at least some part of it) differed from the natural one in its higher ability to leaching. Dependence on the 238U/235U ratio value on leaching conditions testified that the mobile uranium both of natural and fuel origin is not completely transferred into a solution. In such a case a dynamic equilibrium should be established between the solid and liquid phases of the system. Subject to the changes in the physico-chemical leaching conditions such an equilibrium should be disturbed resulting in changes in the uranium isotopic composition of the leachate solution.
To estimate an effect of oxidation of the liquid phase upon the displacement of uranium isotopic composition oxidation of the aliquot part of the leachate solution was performed with persulphuric ammonium in presence of Ag+ cations. When uranium was leached from the soil by hydrochloric acid, evaporation of the solution aliquot, conversion of the salt residual into NO3-form (removing of Cl- ion traces) and its dissolution in 1 M HNO3 preceded to the oxidation operation.
Values of 238U/235U ratio in leachate solutions before and after oxidation of uranium are presented in Table 3. In the case of oxidation of the solution uranium in the leachate solution was more enriched with 235U isotope in comparison with unoxidized solution. The minimal detected 238U/235U ratio reached 2.7, that corresponded to ~ 27% enrichment by 235U.
Table 3. Isotopic composition of uranium in leachate solutions before and after oxidation of uranium.
|
No |
Sampling point |
Leaching agent |
Soil |
Solution without oxidation |
Oxidized solution |
|||
|
238 U/235U |
235 U, % |
238 U/235U |
235 U, % |
238 U/235U |
235 U, % |
|||
|
1 |
Cooling pond |
8 Ì HNO3 |
44.82 |
2.18 % |
28.42 |
3.4 % |
11.74 |
7.85 % |
|
2 |
Yanov railway station |
2 Ì HNO3 |
52.6 |
1.86 % |
40.61 |
2.4 % |
12.17 |
7.6 % |
|
3 |
v. Yanov |
6 Ì HNO3 |
62.8 |
1.57 % |
48.4 |
2.02 % |
20.32 |
4.69 % |
|
4 |
v. Leliv |
7 Ì HCl |
77.5 |
1.27 % |
47.9 |
2.04 % |
23.68 |
4.05 % |
|
5 |
v. Leliv |
2 Ì HNO3 |
77.5 |
1.27 % |
51.1 |
1.92 % |
31.22 |
3.1 % |
|
6 |
Paryshev channel |
7 Ì HCl |
93.8 |
1.05 % |
15.64 |
6.0 % |
2.744 |
27 % |
|
7 |
Paryshev channel |
8 Ì HNO3 |
95.4 |
1.04 % |
61.4 |
1.6 % |
18.93 |
5.0 % |
Experiments to estimate the contents of uranium valency forms in the leachate solutions were also carried out. U(VI)/U(IV) ratios were found to vary from 0.6 to 1.7 [2].
In the experiments on absorption of uranium from hydrochloric acid solutions by the anion-exchange resin AV-17, the authors of the work [2] have found the anionic form that is characterized to U(V1) to be enriched by 235U isotope relatively to U (1V) form.
In the other series of experiments, processes of leaching of fragmented radionuclides as well as fuel uranium from the contaminated soils from left-bank flood-plain of the Prypiat river were studied. Soils were sampled in the right bank of Murovka channel on 20.03.91. By this moment solid-phase fallouts underwent destruction to some extent. (see Part 3).
The levels of surface contamination by 90Sr in the sampling site were from 3.7 to 7.4 MBq/m2. Radionuclide compositions of the studied samples are presented in Table 4.
Table 4. Radionuclide composition of soil samples from left-bank flood-plain of the Prypiat on 20.04.91 [3].
|
Sample |
Radionuclide content, kBq/kg |
||||
|
144 Ce |
106 Ru |
137 Cs |
134 Cs |
90 Sr |
|
|
1 |
57.4 |
18.1 |
134 |
13.7 |
154 |
|
2 |
8.5 |
2.5 |
18.2 |
1.82 |
15.7 |
|
3 |
16.2 |
3.0 |
34.6 |
3.57 |
38.3 |
Gamma-spectrometric analysis and radiochemical determination of 90Sr content were carried out from the same sample. Then the samples were sequentially treated by water with natural hydrochemical composition, 1M solution of ammonium nitrate, and concentrated acid solutions. 90Sr and uranium contents and uranium isotopic composition were determined in each of leachate-solutions. Solid to liquid phases ratio was 1:1, the mixture was stirred for 24 hours, then separation of the phases on the Buchner funnel with membrane filters of 0.2 m m in diameter was carried out. Acid leaching was carried out in teflon dishes under light heating and periodical stirring during 24 hours.
Stringent regime of acid leaching resulted in dissolution of uranium matrix and in partial or complete breakdown of natural uranium-containing minerals, but, because of low uranium concentrations, the applied procedure did not make available determination of its content in a number of leachate-solutions (Table 5). High 235U contents were found in water, salt and acid leachate-solutions. Insoluble residue from the sample 2 after mineral breakdown by hydrofluoric acid was the mixture of accessories (mainly zircon-rutile) and graphite particles. Presence of 90Sr pointed to the definite influence of graphite fuel-containing particles on the uranium isotopic composition.
Table 5. Uranium isotopes in the leachate-solutions from the soils [3].
|
Agent |
Sample 1 |
Sample 2 |
Sample 3 |
||||||
|
238 U/235U |
U, m g/g |
90 Sr, %* |
238 U/235U |
U, m g/g |
90 Sr, %* |
238 U/235U |
U, m g/g |
90 Sr, %* |
|
|
H2O |
10 (9.1%) |
- |
3.0 |
- |
- |
1.7 |
22 (4.3%) |
- |
1.9 |
|
NaAc |
1.4 (41.6%) |
- |
4.2 |
22 (4.3%) |
5.4 × 10-4 |
4.5 |
3.3(23.2%) |
- |
3.3 |
|
6N HNO3 |
36 (2.7%) |
0.39 |
91.7 |
110(0.9%) |
- |
91.4 |
30 (3.2%) |
0.015 |
84 |
|
HNO3+ HCl |
45 (2.17%) |
0.11 |
1.05 |
||||||
|
HNO3+ HF |
2.4 |
||||||||
|
6N HNO3 |
9.9 |
||||||||
* 90Sr content is given as percentage of bulk activity in the soil
In the leachate-solution from the mineral concentrate [15], sampled at the cooling-pond bank, mass contents of heavy nuclides were determined by the mass-spectrometer ”ČPlasma Quad”É (”ČVG Instruments”É) with ionization of the material in inductively connected plasma. The relative contents of masses from 233 to 242 were the next: 233 - 6.29; 234 - 45.1; 235 - 2712.2; 236 - 455.69; 237 - 5.54; 238 - 237999; 239 - 112.61; 240 - 9.68; 241 - 9.11; 242 - 8.38. Ratio of mass of 235 to 238 corresponded here to the enrichment of uranium with 235U isotope up to 1.126%, that is close to ”Čmean”É composition of ejected fuel. In groundwater in the ”ČRed Forest”É near the Chernobyl NPP, the presence of uranium with mass ratios 238U/235U from 50 to 54 was fixed in 1987 [16]. It corresponds to 1.8-2.0% enrichment of the fuel.
Comparison of the data from the cited works makes it evident that the intervals of variations of uranium isotopic composition in water phase in its leaching from soils in natural conditions and in the laboratory are actually identical.
””
5. Leaching of nuclides from dispersed fuel of graphite composition.
Ability of uranium and fission products to leaching from hot particles of predominantly graphite composition sampled in the premises of the ”ČSarcophagus”É was studied in the work [3]. Radionuclide composition of sampled particles varied noticeably. 90Sr content in some particles varied from 37 to 814 kBq/g, that of 137Cs - from 2 to 12 MBq/g. Radioactivity of a number of particles was caused exclusively by nuclides of 134Cs and 137Ñs. These data testified predominantly condensation genesis of the particles.
Leaching of radionuclides from graphite particles was carried out in the hermetically sealed vessels under continuous stirring. After set time expiration solid phase was separated on the membrane filter of 0.2 m m in diameter and then was treated by the water or acid solution again to continue the process of leaching. Sequential effect of mineral acids HF and HCl in different conditions, including under high temperature (200 0Ñ) was carried out in the hermetically sealed teflon cylinder.
Nuclide composition of leachate-solutions from graphite hot particles was determined by mass-spectrometer ”ČPlasmaQuad”É (”ČVG Instruments”É). Solution of the natural uranium has a strictly constant 238U/235U ratio equal to 137.88. A series of 10 measurements showed the 238U/235U ratio in 138.6 ± 2.0 when the time of the single measurement was 3 min. Uranium content and its isotopic composition were determined in the experimental leachate solutions from hot particles.
Experimental results are presented in the Tables 6 and 7. Experimental results testify the difference in the rates of dissolution of strontium, cesium, and uranium nuclides in the process of their leaching from graphite particles and their independence (Fig. 3). It is explained by authors by the distinctions of formation of uranium-containing particles: recrystallization of uranium compounds following the ”Črelease”É of fragment radionuclides took place under conditions of effects of high temperatures and interaction between construction materials and air oxygen.
Table 6. Extent of radionuclides leaching from the graphite hot particles in the experiment with subsequent treatment with water and mineral acids [3].
|
Treatment |
Time of exposition, hours |
Total leaching, % |
|||
|
total |
for separate fraction |
90 Sr |
137 Cs |
U |
|
|
Water 1 |
1 |
1 |
32.7 |
75.7 |
69.0 |
|
Water 2 |
4 |
3 |
52.9 |
89.3 |
69.1 |
|
Water 3 |
7 |
3 |
66.4 |
92.4 |
69.2 |
|
Water 4 |
13 |
6 |
75.1 |
93.5 |
79.6 |
|
Water 5 |
23 |
10 |
85.7 |
94.5 |
80.3 |
|
Water 6 |
40 |
17 |
90.5 |
95.6 |
80.5 |
|
Water 7 |
67 |
27 |
94.3 |
96.0 |
- |
|
HNO3 |
** |
** |
** |
** |
99.1 |
|
HF |
** |
** |
** |
** |
99.75 |
|
HCl |
** |
** |
** |
** |
99.98 |
** Has not been controled
Table 7. Uranium isotopic composition in water leachate-solutions from the graphite hot particles [3].
|
Conditions of leaching |
Isotope ratios |
|||||
|
Time, hours |
Leaching agent |
235 U, % |
234 U/238U |
238 U/235U |
236 U/238U |
|
|
1 |
H2O |
1.078 |
1.6 × 10-4 |
91.70 |
1.8 × 10-3 |
|
|
3 |
H2O |
1.088 |
2.3 × 10-4 |
90.91 |
1.8 × 10-3 |
|
|
6 |
H2O |
1.107 |
1.6 × 10-4 |
89.29 |
1.9 × 10-3 |
|
|
10 |
H2O |
1.068 |
1.5 × 10-4 |
92.60 |
1.8 × 10-3 |
|
|
17 |
H2O |
1.099 |
1.4 × 10-4 |
89.96 |
1.9 × 10-3 |
|
|
27 |
H2O |
1.088 |
2.5 × 10-4 |
90.91 |
1.6 × 10-3 |
|
| ”” |
HNO3 |
1.010 |
1.5 × 10-4 |
97.96 |
1.6 × 10-3 |
|
| ”” |
HF |
1.906 |
2.6 × 10-4 |
51.47 |
1.7 × 10-3 |
|
| ”” |
HCl |
1.986 |
2.4 × 10-4 |
49.36 |
1.7 × 10-3 |
|
|
Natural uranium |
0.721 |
6 × 10-5 |
138.6 |
<2 × 10-6 |
||
(*) Table was drawn up after [3], % 235U was calculated for the convenience of discussion.
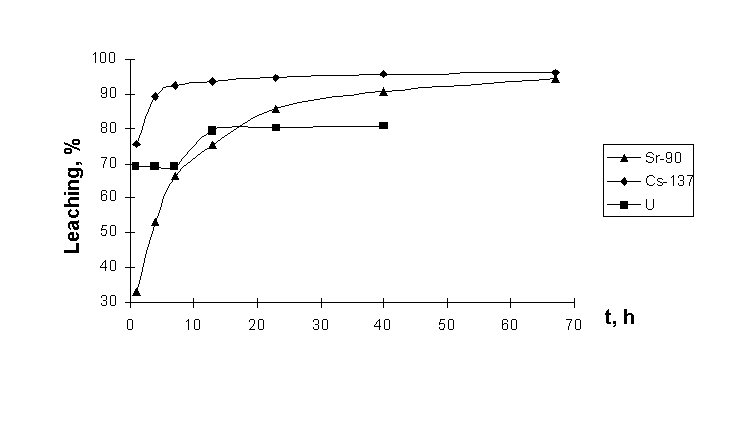
Fig. 3. Kinetics of leaching of 137Cs and 90Sr radionuclides, and fuel uranium from the graphite hot particles [3].
””
Isotopic composition of uranium in sequential water leachate-solutions from graphite particles was uniform varying in ranges of 1.07-1.11% enrichment, which was rather close to calculated values for the bulk mass of the fuel. Only insignificant part of it, i.e. the most resistant to dissolution, corresponded to 2% enrichment.
Authors of the work [3] distinguish two groups of uranium-containing phases in graphite particles by their resistance to action of leaching agents:
- depleted fuel with 235U content about 1%; it constitutes the main mass (~ 99%) of uranium that is contained in the particles and dissolves readily in the water and nitric acid;
- uranium-containing phase with 238U/235U isotopes ratio corresponded to that of the ”Čfresh”É 2% reactor fuel; its content is quite insignificant, and its dissolution requires high temperature and pressure.
The both phases contain 236U, formed in result of nuclear processes.
””
6. Hypothesis for fractionation of uranium nuclides in its leaching from fuel-containing soils.
Heterogeneous composition of uranium-containing compounds should be taken into account in an attempt to explain the results of determination of uranium isotopic composition in the soils contaminated by solid-phase radioactive fallouts. The contaminated soils may be expected to contain the next uranium-containing compounds:
- natural minerals and soil compounds, containing uranium with composition of about 0.7 % 235U;
- technogeneous fuel-containing particles with uranium of about 1.1% 235U composition;
- technogeneous fuel-containing particles containing uranium of fresh fuel charged immediately before the accident and enriched up to 2% of 235U; statistical expected contribution of that particles is less than 1% of technogeneous uranium.
Relationship between technogeneous and natural uranium in the studied soils increases with approaching near the source (Chernobyl NPP Industrial site ) up to their comparable contents [1, 15]. 235U contents could not exceed in the most contaminated samples 1.1% (238U/235U ratio was about 89.5). Part of 235U in slightly contaminated soils should be close to 0.7%.
According to work [1] 238U/235U ratio in the water phase after extraction of uranium from contaminated soils varied from 126 to 40 (Table 2). The most significant enrichment by 235U isotope (up to 2.4%) was reached in the soil sampled directly near the IV unit. Differences between expected isotopes ratios and those obtained experimentally exceeded substantially the error of mass-spectrometric analysis. Finding of high-enriched uranium presents the presumption that isotopic heterogeneity of nuclear fuel in the IV unit existed before the accident.
Isotope fractionation in leaching of uranium observed in the work [1], confirmed by the work [15]and probably by the other one [6], showed that correspondence of uranium isotopic composition in the solution to that of the soil could be reached provided that the analyzed element was completely dissolved. But hot particles are rather resistant to action of acids. Complete dissolution of hot particles was observed only after roasting of the samples at 650 0Ñ with subsequent treatment with nitric acid at heating or with mixture of chlorate and hydrofluoric acids [2]. In the cited work [1] completion of uranium extraction into liquid phase was not qualified, so statement of uranium isotopic composition in the solid phase (in soil) presented in Table 2 was not quite correct. However, this circumstance does not influence upon basic results of experiments on leaching of uranium from contaminated soils that testify the possibility of fractionation of uranium isotopes 235U and 238U between solid and liquid phases.
Factors of separation may be used for numerical representation of fractionation effects. If 235U and 238U contents in the mixture of uranium isotopes are expressed in mole parts, the relative isotope contents in the solid and liquid phases would be presented as follows [16]:
R= x /(1 - x) ,
where: x is the mole part of 235U isotope, and (1 - x) is the mole part of 238U isotope.
Coefficient of the single separation is given by the equation:
a = R*/ R ,
where the index * is attributed to the liquid phase.
Factors of separation calculated on the data of Table 3 are presented in the Table 8. Presence of any other uranium isotopes which contents did not exceed 0.3% by mass, was not taken into account in calculation of the mole parts of 235U and 238U isotopes.
Table 8. Factors of separation of uranium isotopes between solid phase and solution in its leaching from the soil.
|
No |
Sampling site |
Leaching agent |
R 235U in soil |
Solution |
|||
|
without oxidation |
after oxidation |
||||||
|
R*1 |
a 1 |
R*2 |
a 2 |
||||
|
1 |
Cooling pond |
8 Ì HNO3 |
0.0223 |
0.035 |
1.58 |
0.085 |
3.82 |
|
2 |
st. Yaniv |
2 Ì HNO3 |
0.019 |
0.0246 |
1.29 |
0.082 |
4.32 |
|
3 |
v. Yaniv |
6 Ì HNO3 |
0.016 |
0.0206 |
1.29 |
0.049 |
3.09 |
|
4 |
v. Leliv |
7 Ì HCl |
0.013 |
0.021 |
1.61 |
0.042 |
3.27 |
|
5 |
v. Leliv |
2 Ì HNO3 |
0.013 |
0.019 |
1.51 |
0.032 |
2.48 |
|
6 |
Paryshev channel |
7 Ì HCl |
0.0106 |
0.064 |
6.0 |
0.36 |
34.2 |
|
7 |
Paryshev channel |
8 Ì HNO3 |
0.0105 |
0.016 |
1.55 |
0.053 |
5.04 |
Factors of separation of uranium isotopes in leaching of uranium from different soils by the acids are in the range 1.3 - 1.6; in leaching by the same but oxidated solutions the factors vary in the range 2.5 - 5. Result of uranium leaching from the soil sampled on the bank of Paryshev channel (Table 8, No. 6) was the exception. Fractionation of isotopes in oxidation with 7M HCl in this case exceeded by an order of magnitude the effects of the rest experiments. Authenticity of the more high separation of isotopes in leaching by oxidated solutions was confirmed by the results of independent experiments on determination of isotopic compositions of the valent forms of uranium [2], which showed enrichment of U(VI) form by 235U isotope.
Different mobility of 235U and 238U isotopes caused by their unequivalent energetic position in the solid phase should be assumed to explain satisfactorily the fractionation of uranium isotopes: nuclides with the lower energy of bond in the solid phase pass predominantly into liquid phase.
It is still unclear which of the uranium-containing fractions in soil has unequivalent energetic bonds of 235U and 238U isotopes, and how that difference could be generated.
Since fractionation of uranium isotopes in leaching from natural minerals usually does not take place, so observed isotope separation in the experiments is due to presence of technogeneous uranium of dispersed fuel (solid-phase fuel-containing fallouts, formed in result of the IV unit accident). Comparison of the results of leaching of uranium from graphite particles (Table 7) and that from the soils (Tables 3 and 5) shows the difference in the isotope enrichment of uranium. Fuel-containing fragments of graphite particles formed through condensation of evaporated fuel did not exhibited practically the isotope fractionation in leaching. Rebuilding of the structure took place in recrystallization of uranium. Defects of the lattice that was characterized by the difference in the mobilities of uranium isotopes were eliminated, even if they existed in the initial substrate. Thus, only the particles formed by mechanical fragmentation of the fuel can contain uranium with different mobility of isotopes.
Two kinds of the particles, therefore, can be distinguished by the uranium isotopes mobilities in the dispersed fuel formed as the result of the Chernobyl accident. They are the particles formed by mechanical fragmentation of tablets and the particles formed in recrystallization of the fuel.
It enables to assume the presence of at least two beings of uranium in irradiated dispersed fuel:
1) Uranium of irradiated fuel in products of fragmentation of the fuel - particle of indefinite habitus. It may be partially oxidated. Uranium isotopic composition is close to 1-2% enrichment.
2) Uranium of irradiated fuel in form of spherical or fiber-shaped particles that undergone the recrystallization in result of evaporation and condensation. Recrystallization fuel uranium as a part of graphite particle may be in oxide form or probably metallic uranium. Isotopic composition should be close to 1% enrichment, and insignificant part of this uranium has isotopic composition close to 2% enrichment.
Fractionation of uranium isotopes between liquid and solid phases is not observed in exposure of the second type fuel particles to water solutions. When the fuel particles of the first type are exposed to water solutions, uranium in liquid phase may be enriched by 235U isotope relative to the uranium of the solid phase exposed to leaching.
What is the reason of different mobility of 235U and 238U isotopes in dispersed fuel? Two hypotheses are worth an attention.
The first one supposes heterogeneous composition of nuclear fuel of RBMK reactor consisting of fractions with different enrichment by 235U, uranium isotopes of the more enriched fraction having higher mobility due to features of preparation technology.
Another hypothesis is based on the assumption that selective loosening of bonds of separate 235U atoms takes place in the solid body under action of slow neutrons, to which 235U nuclei have relatively large values of capture cross sections. Energy of the nuclei in excited state is probably sufficient to loosening of the chemical bonds of 235U in the solid phase for a long time. In this case the probability of the process of excitation of 235U nuclei due to reversible absorption of neutrons without fission is yet uncertain. It is also not clear whether the excitation of 235U nuclei takes place in a standard mode of operation of RBMK reactor, or it is somehow connected with the emergency state of the IV unit of the Chernobyl NPP.
Acknowledgments: the authors are grateful to prof. V.Barjakhtar for helpful discussion of the nature of fractionation of the uranium isotopes, to Yu.Olkhovick and Yu.Slupizcky for giving some unpublished data and to L.Kononenko for help in editing the paper. Authors also thank Dr.T Imanaka and O.Nasvit for their attention to this work.
””
BIBLIOGRAPHY
1. Sobotovich E. V. , Chebanenko S. I. Isotopic composition of uranium in soils of near zone of Chernobyl NPP // Docl. AN SSSR. - 1990. - ? 4. - p. 885-888 ( in Russian).
2. Sobotovich E. V. , Bondarenko G. N. , Olkhovick Yu.Ŗ¢. et. al. Radiogeochemistry in zone of influence of Chernobyl NPP. - Kiev, Naukova dumka, 1992. - 144 p. ( in Russian).
3. Sobotovich E. V. , Olkhovick Yu.Ŗ¢., Koromyslichenko T. I. et. al. Plumbago hot particles from Sarcophagus of Chernobyl NPP // Dokl. AN Ukraine. - 1993. - ? 11. - p. 170-174. ( in Russian).
4. Chernobyl Disaster. -Ed. by V.Barjakhtar. - Kiev. -Naukova dumka.-1996.-575 p. (in Ukrainian).
5. Gerasimov A.S., Zarizchkaj T.S., Rudik A.P. Reference book of formation of the nuclides in the nuclear reactors. Energoatomizdat, M. , 1989. ? 575 p. ( in Russian).
6. Kuzmina I.Ŗ©., To«“«¢revs«“ij V.V. Destruction of nuclear fuel after disaster in Reactor No 4 of Chernobyl NPP // Obninsk, 1996. - FEI-231.
7. Begichev S.N., Borovoj A.A., Burlakov V.V. et al Fuel of reactor of 4 unit of Chernobyl NPP. -ŖĄ., 1990«Ŗ., -21p.- -(preprint Atomic energy Institute; 5268/3). ( in Russian).
8. Belyaev S.T., Borovoy A.A., Demin V.N. et al: The Chernobyl Source Term. Radiation protection-53. Proceedings of Seminar on Comparative Assessment of the Environmental Impact of Radionuclides Released during Three Major Nuclear Accidents, Commission of the European Communities, Luxembourg, 1-5 October 1990, v. 1, Report EUR 13574.
9. USSR State Committee on the Utilization of Atomic Energy ”ČThe Accident at the Chernobyl NPP and its Consequences”É IAEA Post Accident Review Meeting, Vienna, 25-29 August 1986.
10. Kuzmina I.E. Experimental Research into the Content of Particle Releases from the Sarcophagus. - In: Proc. Symp. ”ČSarcophagus Safety`94”É, Chernobyl, Ukraine, March, 1994. OECO Documents - 1995, France: Nucl. En. Ag. Org. for economic co-operation and Development. 850 p., p. 409-418.
11. Kuzmina I.E., Lobach U.N. Nuclear fuel and peculiarity of formation of aerosols of the Sarcophagus // Ŗ¢tomnaja Energija. - 1996. ( in Russian).
12. Bondarenko G.N., Dolin V.V., Valter Ŗ¢.Ŗ¢. et al : Composition and structure of solid-phase carriers of activity in soils of the exclusion zone of Chernobyl NPP // Problems of Chernobyl exclusion zone, v. 2.- Ŗ“iev, Naukova dumka, 1995, p. 147-152. ( in Russian).
13. Sobotovich E. V. , Valter Ŗ¢.Ŗ¢., Olkhovick Yu.Ŗ¢., et al. Solid radioactive fallouts in Chernobyl exclusion zone // Dokl. AN Ukraine. - 1993. - ? 8. - c. 173-177. ( in Russian).
14. Bondarenko G.N., Kononenko L.V. The kinetics of transformation of 90Sr and 137Cs forms in soils // Mineralogicheskij Jurnal.-1996.- v.18.- No. 3.- p. 48-57. ( in Russian).
15. Olkhovick Yu.Ŗ¢. et al. Report RGE NACU, Kiev, 1993 ( in Russian).
16. Shemlj M., Perje G. Separation of isotopes. ŖĄ.: Ŗ¢tomizdat, 1980. - 169 p. ( in Russian).
””
””