Our lab is Donated Fund Laboratory (by Asahi Vision Inc.) on Research Reactor Institute, Kyoto University since 2017.
Purpose:
We study the significance of D-amino acids existence in aged tissue and relationship with age-related diseases. Proteins consist exclusively of L-amino acids; nonetheless, D-aspartic acid (D-Asp) has been detected in various human tissue such as tooth, bone, aorta brain, and eye lenses from elderly individuals. In such tissue component proteins, there are a lot of isomerization site of Asp residues. We have identified many isomerization sites of α-crystallin from cataractous lens, while pathogenesis has been still obscure. One goal of our study is to relate isomerization of Asp residues with age-related dieaseses. Another goal is developping methhodology for detecting isomerization more sensitive, rapid and quantitatively. The presence of D-Asp in aged tissues of the living body has been explained as a result of racemization of aspartyl residues in the protein over time. Thus, It could be the useful tool for detecting the disease at molecular levels. We are now focusing on both the characterization of D-amino acid, followed by developping the instrument for detecting those at very early phase during life. Our ongoing project is introduced at the bottom of this page.
D-amino acid introduction:
Before the emergence of life, it is considered that energy for the synthesis of DL-amino acids came from various sources on the primitive earth, such as ultraviolet light, electric discharges, etc. Although the chemical and physical properties of L-amino acids and D-amino acids are extremely similar, only the L-amino acids were selected for polymerization and formation of peptides and proteins on the primitive earth. Nobody knows why, where and how nature selected L-amino acids, or whether the selection of L-amino acids had a logical reason or was a chance occurrence. However, it is clear that only one of the enantiomers could be selected because polymers which consist of many diastereoisomers of amino acids would not be able to be folded into a proper structure like a protein. Therefore, “homochilarity" is essential for life. However, D-amino acids were recently detected in various living organisms in the form of free amino acids, peptides, and proteins. It has been well known D-amino acid in food and medicine or opioid peptide, while not in protein. The mechanism and roles of D-amino acid formation has been still obscure in life. However, presumably D-amino acid formation in homo-chiral protein would alter the tertiary structure of protein, lead partially misfolding and cause abonormal protein function in aged tissues. It could be a new target for improving quality of life. Inhibition of D-amino acid formation would surppress age-related diseases and delay molecular level aging. We hope to clear the meaning of D-amino acid in life and open the new science as “parachilality" in the world.
Cataract:
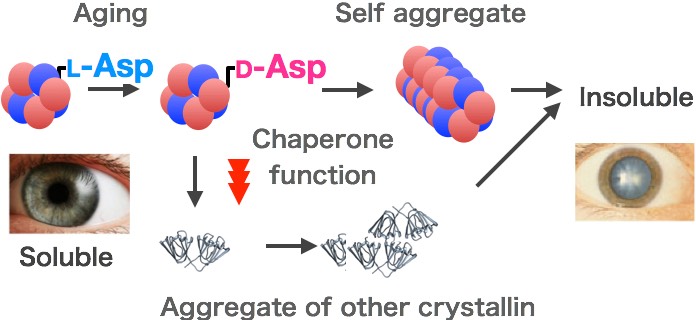
Most of all people will have cataract in late part of life. The aged cataract formation is caused by the accumulative precipitation of lens proteins incorporating diverse post-translational modifications during the aging process. Such modifications decrease crystallin solubility, lens transparency, and ultimately lead to the development of a cataract. One of the crystallin superfamily, α-crystallin is a large molecule that consists of two kinds of polypeptide: αA and αB. α-crystallin has chaperone-like activity that inhibits the aggregation of other crystallins. The crystallin aggregation and insolubilization are caused by non-enzymatical amino acid modifications. One such modification, isomerization of Asp, was our target described as above. Each type of Asp isomerization increased in an age-dependent manner and site-specifically. The recent studies by our LC/MS/MS analysis have shown that huge amount of aspartyl residues (Lα-Asp) inverted isomers (Lβ-Asp, Dα-Asp, Dβ-Asp) in abnormal crystallin from insoluble fractions of aged lens. Therefore, we are now investigating about what is caused by isomerization in crystallins and how does isomerization cause lens crystallin insolubilization to clear the cataract pathogenesis.

On going project:
1.
Developing Non-invasive methodology to detect protein aggregation at very early phase of
age-related diseases.
2. Anti-aging study using D-amino acids as a biomarker.
3. Identification of D-amino acid containing protein in various aged tissues.
4. Developing the rapid and comprehensive methodology to analyze D-amino acid in proteins using with
mass spectrometry (LC-MS/MS-MRM).
5. The study about relationship between D-amino acid formation and other modification in protein,
triggering age-related diseases.
6. Study of the effect from ultraviolet and gamma ray irradiation on protein aggregation.
7. Establisghment new biomarker; D-amino acid containing peptide (DAP) in blood.