Neutron Material Science
Professor
- OKUCHI, Takuo e-mail: okuchi<atmark>
Associate Professor
- ARIMA, Hiroshi e-mail: arima.hiroshi.3r#kyoto-u.ac.jp
Assistant Professor
- UMEDA, Yuhei e-mail: umeda.yuhei.2e#kyoto-u.ac.jp;
* replace <atmark> with @rri.kyoto-u.ac.jp, # with @
Graphite and diamond show entirely different physical properties such as electric conductivity and mechanical hardness. While both of these materials are made of carbon atoms only, their atoms are arranged in space very differently. We aim to understand the origin of such different properties in variety of crystalline and amorphous materials, by analyzing atomic level structure and dynamics, that is, where atoms are and how they are moving in very high resolution in space. Using state-of-the-art quantum beam methods such as neutron diffraction and scattering (including pulsed sources), X-ray diffraction and scattering (including free electron laser sources), as well as electron beam analysis in a complementary manner, we analyze where atoms are and how they are moving in energy materials, functioning materials, as well as in materials in the solar system and the earth. The arrangements of atoms in space dimension are determined by diffraction methods, while the motion of atoms in time dimension are determined by inelastic scattering methods. Applying both of these methods, we find the origin of physical properties of the described materials starting from their atomic-scale behaviors. For making good analysis, our samples are synthesized in the best-suitable synthesis environments including ultrahigh pressure and/or high temperature conditions.
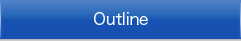
Among the quantum-beam methods, neutron is particularly sensitive for analyzing light elements such as hydrogen and lithium. Therefore, we use this merit for analyzing the roles of light elements in the materials. Figure 1 shows an example of structure analysis of hydrogen inside the deep earth. Figure 2 shows an example of dynamics analysis of hydrogen in anther mineral which shows large proton conductivity at high temperatures conditions.
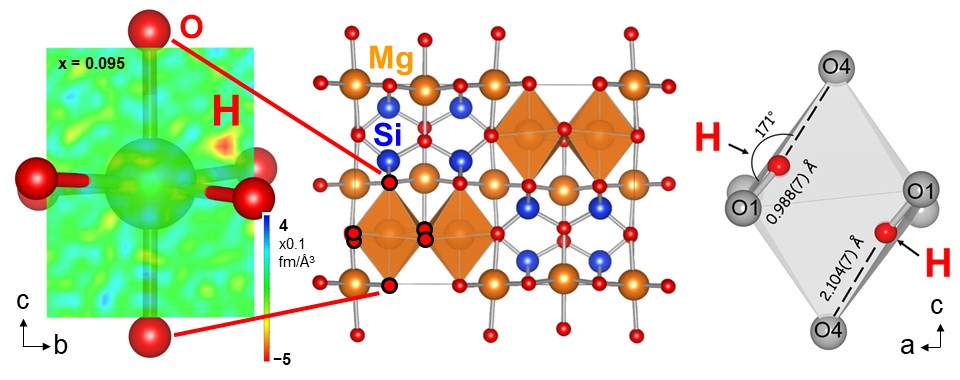 |
| Fig. 1. Structure analysis of wadsleyite [b-Mg2SiO4] synthesized at 170,000 atmospheric pressure, at which condition it actually exists. The neutron scattering length density distribution (left) showed 1500 ppm of hydrogen within the structure, indicating that this hidden mineral inside the deep earth has a capacity of hydrogen three times of the entire ocean mass. |
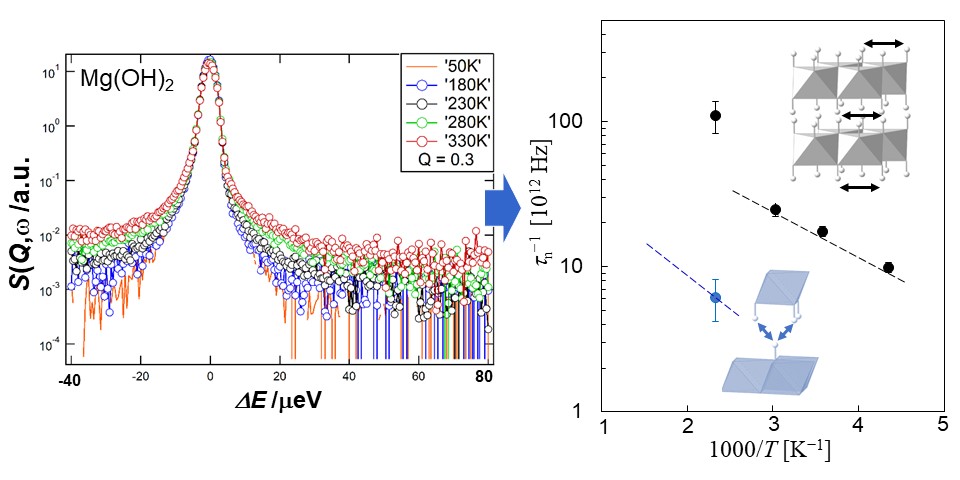 |
| Fig. 2. Quasi-elastic neutron scattering analysis of brucite Mg(OH)2. Increasing width of the broad tail of the energy spectra (left) shows increasing jump frequency of hydrogen within the layered structure (right). Two types of hydrogen dynamics are observed to occur within the same structure (right). |