Biomolecular Structure
Professor
- INOUE Rintaro, e-mail: inoue.rintaro.5w*
Associate Professor
- CHATAKE Toshiyuki, e-mail: chatake.toshiyuki.6x*
Assistant Professors
- KITA Akiko, e-mail: kita.akiko.4u*
- KAWAGUCHI Akio, e-mail: kawaguchi.akio.6s*
◆replace* with @kyoto-u.ac. jp
Biomolecules are molecular machines whose specific structures are related to their functions. Structure determination of biomolecules is essential to reveal the structures and functions of biomolecules. Crystallography is one of the most powerful methods for structure determination, and ~80% of the structures of biomolecules have been determined by X-ray crystallography. Although predictions of protein structure (AlphaFoled) and cryo-EM are increasing, X-ray crystallography still has an advantage in determining the atomic positions accurately. On the other hand, neutron crystallography can determine the positions of hydrogen atoms, because the scattering lengths of hydrogen and its isotopes are comparable to those of non-hydrogen atoms. In our laboratory, X-ray and neutron crystallography have been used complementarily.
1. D/H contrast neutron protein crystallography
The neutron scattering lengths of hydrogen and deuterium are different (bH = 3.8 fm, bD = 6.6 fm). The difference between the neutron Fourier maps of H2O-solvent and D2O-solvent protein crystals provides density contrasts of D/H exchangeable atoms (D/H contrast) of amino and hydroxyl groups in protein and water. D/H contrast neutron protein crystallography is expected to improve the efficiency and accuracy of hydrogen analysis. In our laboratory, the algorithm for calculating the D/H contrast in real space has been developed, and D/H neutron crystallographic analyses of proteins have been carried out.
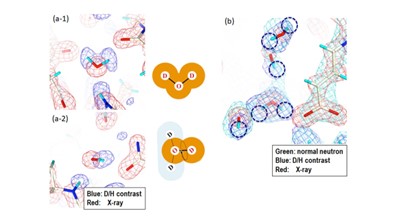
Fig. 1. Neutron D/H contrast map in the solvent region of a protein crystal. (a) Two types of water molecules with different dynamic behavior. (b) Neutron contrast map superimposed on conventional neutron map.
2. Structural study of novel or biologically interested protein
To know the protein structures including H atoms by neutron diffraction method as described, it is necessary to determine their three-dimensional structures with no H atoms. We are carrying out structure analyses of novel or biologically interested proteins with collaborators by X-ray diffraction method, that has no upper of lower limits of the molecular weight.
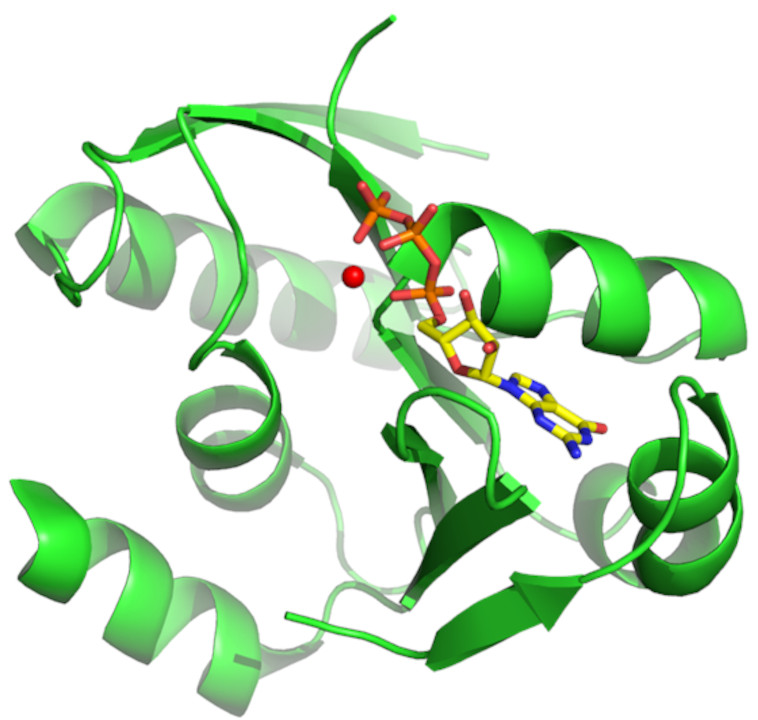
Fig. 2. GTP-dependent dephospho-coenzyme A kinase from the hyperthermophilic archaeon, Thermococcus kodakarensis. (Collaborative work with Prof. Atomi @Kyoto University, Graduate School of Engineering)
3. Structural study of water-soluble vitamin K2 derived from natto
Natto contains the water-soluble complex (natto-MK-7) of fatty-soluble vitamin K2 (MK-7) and peptides (KBF: K-binding factor). Vitamin K is thought to contribute to bone and cardiovascular health. The solubility of natto-MK-7 in water would be advantageous in nutritional, medical and pharmaceutical applications. In our laboratory, purification and structural analysis of natto-MK-7 have been proceeded to reveal the structures of KBF and natto-MK-7.
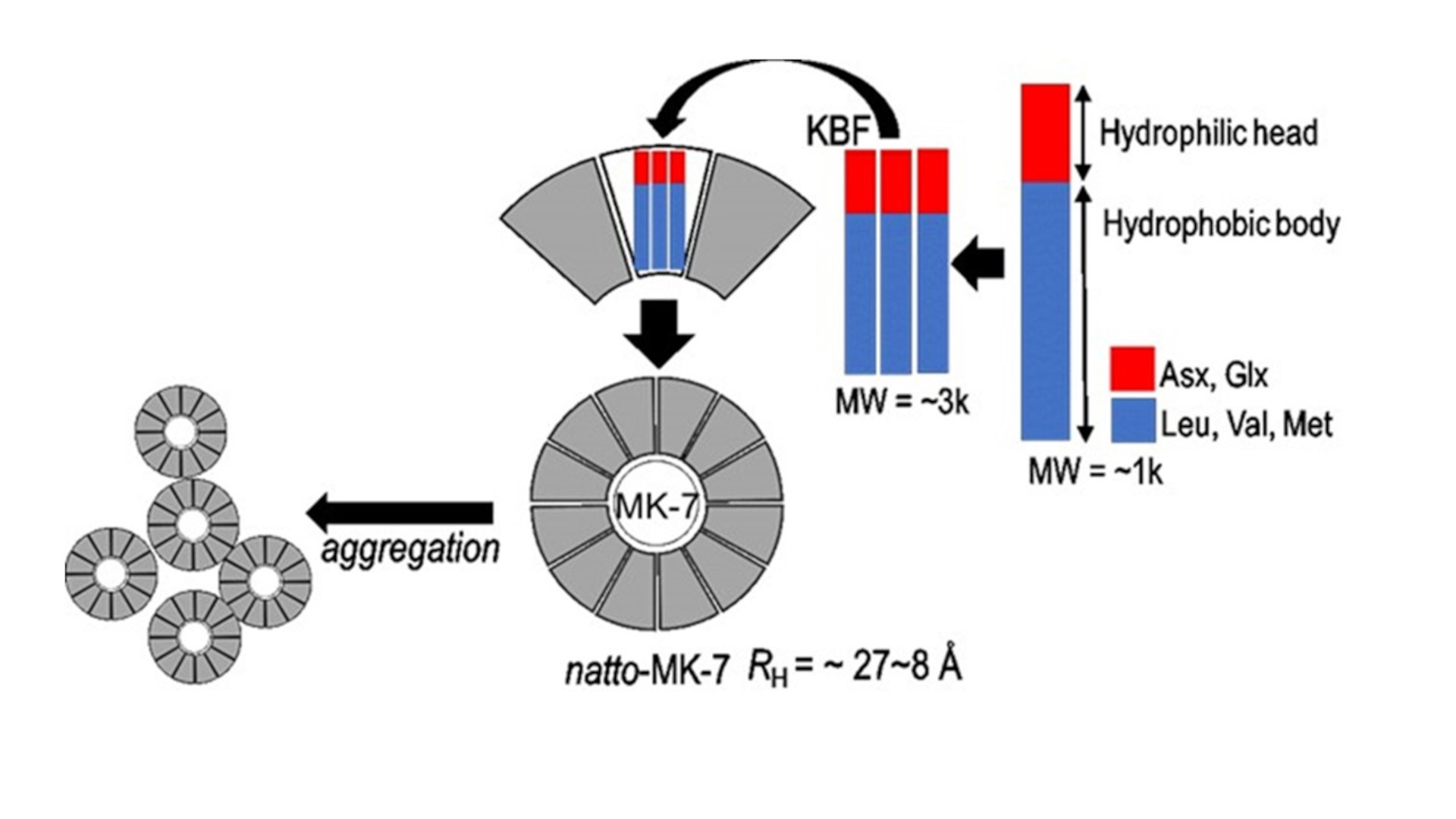
Fig. 3. The structural model of natto-MK-7