Radiation Biochemistry
Professor
- TAKATA, Takumi e-mail: takumi<atmark>
Lecturer
- KINOUCHI, Tadatoshi e-mail: kinouchi<atmark>
* replace <atmark> with @rri.kyoto-u.ac.jp
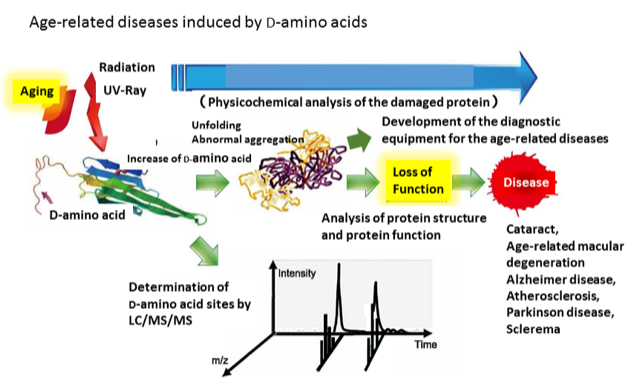
Although the chemical and physical properties of L-amino acids and D-amino acids are extremely similar, only L-amino acids were selected for the polymerization and formation of proteins on the primitive Earth. There were gases, heat, water vapor and extremely intense irradiation before the origin of life. However, nobody knows why, where, or how nature selected L-amino acids as the building blocks of proteins for life. “To be simple” maybe the better reason. It is clear that only one of the enantiomers could be selected, because polymers consisting of many diastereomers of amino acids could not be folded into a proper structure. Therefore, homochirality of amino acid in protein is essential for life. On the other hand, recently, numerous D-amino acid residues have been identified in misfolded protein taken from aged tissues.
Protein misfolding is believed to be the cause of numerous age-related diseases, such as cataracts, Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease. Although the exact reason is unknown, protein misfolding in such tissues may be triggered by an accumulation of spontaneous post-translational modifications (PTMs) of amino acid residues in pathogenic protein. The typical PTMs are oxidation, truncation, deamidation, racemization and isomerization of amino acid residues. We focus on the PTMs, especially the racemization of aspartate residues (Asp) from L-Asp into D-Asp (including isomerization of each -linkage) in misfolded proteins, and the relationship to age-related diseases. This is the so-called breakdown of homochirality of amino acid residues in proteins. Although we have identified many D-Asp (with D--Asp as the result of isomerization) of aged proteins, their pathogenesis has been obscure.
One objective of our study is to relate the isomerization/racemization of Asp residues to age-related diseases. Another objective is to develop a methodology for detecting isomerization in a more sensitive, rapid, and quantitative manner. The presence of D-Asp in aged tissues of the living body has been explained as a result over time of non-enzymatic isomerization/racemization of aspartyl residues in the protein. Thus, it could become a useful and generally accepted method of detecting disease at the molecular level and at an early stage. In other words, the inhibition of D-amino acid formation would suppress age-related diseases and delay molecular level aging.
The driving force of the isomerization of Asp residues should be of interest. We hypothesize a contribution from the sequence and structure of protein, especially the initial misfolding of protein due to PTMs such as oxidation and deamidation. To simulate such conditions, we are using a variety of endogenous stresses (for example: heat, reactive oxygen) or exogeneous stresses (for example: UV, irradiation in special situations) for proteins. We have also developed and utilized an original D/L analysis by using LC-MS/MS systems that can detect the amount of Asp isomers and determine their hot spots in aged tissues. We hope to make clear the meaning of D-amino acid in life and open a new science of “para-chirality.”
 |